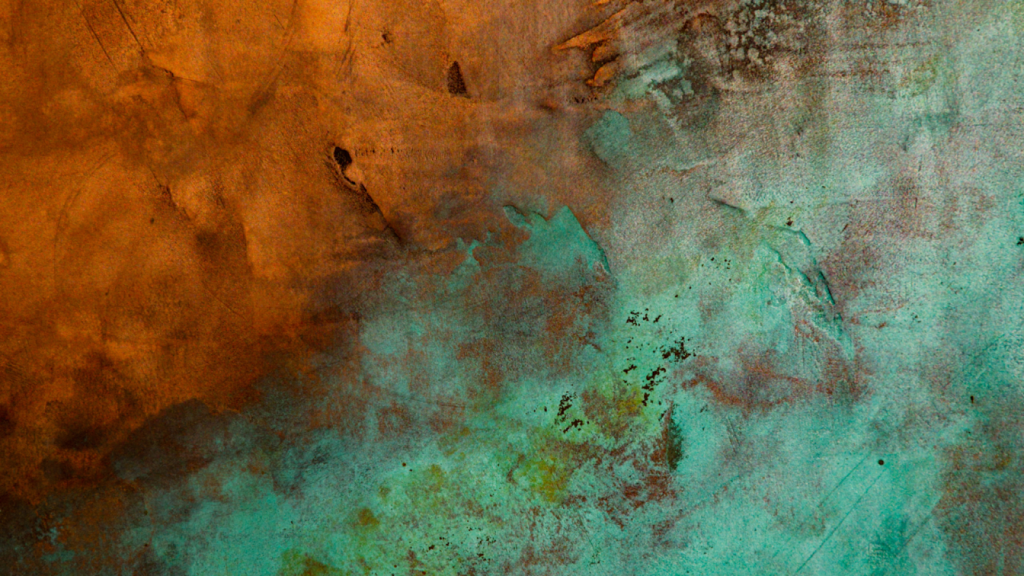Key Takeaways
- Aesthetic Versatility and Durability: Oxidized steel finishes offer a wide range of colors and textures, from warm auburn to deep black, while enhancing the material’s durability and corrosion resistance. This makes them ideal for projects requiring both visual appeal and long-lasting performance.
- Controlled Oxidation Techniques: Mastering controlled oxidation involves managing factors like iron, oxygen, moisture, temperature, and chemical agents. This precision allows for creating consistent, high-quality finishes tailored to specific aesthetic and functional goals.
- Essential Pre-Treatment and Post-Treatment: Effective oxidized steel finishes require thorough pre-treatment, including degreasing, decontamination, and ensuring a uniform surface. Post-oxidation treatments, such as neutralization and protective coatings, help maintain the finish’s integrity and appearance over time.
Within the rigorous discipline of aerospace engineering, material selection and surface treatment processes play a critical role in optimizing performance and ensuring the longevity of critical components.
Oxidized steel finishes have garnered significant attention in recent years due to their unique aesthetic appeal and practical functionality. By employing precisely controlled oxidation techniques, the inherent properties of steel can be manipulated to create surfaces exhibiting enhanced durability, superior corrosion resistance, and even tailored light management capabilities.
In this piece, we will discuss the technical aspects of creating oxidized steel finishes and explore the methods, materials, and best practices that ensure superior results. We aim to offer a comprehensive guide that helps professionals achieve consistent and durable oxidized finishes in their projects.
What is Oxidized Steel?
Oxidized steel is a type of steel that has undergone a controlled chemical reaction with oxygen to produce a layer of oxide on its surface. This process is commonly used in industrial applications to enhance steel components’ durability, aesthetics, and corrosion resistance.
Steel oxidation occurs when the metal’s surface comes into contact with oxygen, typically in the form of air or water. This exposure triggers a series of chemical reactions that convert the iron in the steel into iron oxide. The resulting oxide layer creates a protective barrier on the steel’s surface, helping to prevent further oxidation and corrosion.
The Science Behind Oxidation
The captivating aesthetic of oxidized steel finishes arises from a well-understood chemical process. At the heart of it lies the inherent reactivity of iron (Fe) within steel. In the presence of moisture (H2O) and oxygen (O2), iron readily undergoes oxidation, forming iron oxide (FeO), commonly known as rust.
However, the controlled environment employed in creating artistic steel patinas deviates significantly from the uncontrolled rusting of neglected steel. Here, the focus lies in manipulating and directing this reaction to achieve specific aesthetic and functional goals.
Several key factors come into play:
- Iron (Fe): The primary element forming the desired oxide layer.
- Oxygen (O2): Readily available in the environment, oxygen serves as a crucial reactant in the oxidation process.
- Moisture (H2O): As a catalyst, moisture accelerates the oxidation rate.
By carefully controlling these elements and introducing additional variables, artisans achieve targeted results:
- Time: Extended exposure to the oxidizing environment leads to the formation of thicker oxide layers, resulting in darker finishes.
- Temperature: Elevated temperatures accelerate the oxidation process and can influence the final color palette.
- Chemical Agents: The strategic application of specific chemicals, such as salts or liver of sulfur, can dramatically alter the texture and color of the developing oxide layer.
Mastering the scientific principles governing oxidation empowers metalworkers to achieve consistent and visually stunning results. This fusion of scientific understanding and artistic vision transforms a fundamental chemical reaction into a powerful design tool.
Benefits of Using Oxidized Steel Finishes
Architects, designers, and metalworkers increasingly use oxidized steel finishes for their inherent benefits. Here’s a closer look at the key advantages that elevate this technique:
Unparalleled Aesthetic Flexibility
Oxidized steel surpasses the limitations of a singular color palette. The meticulously controlled oxidation process unlocks a spectrum of captivating finishes, ranging from warm, auburn hues to dramatic, deep blacks. This exceptional aesthetic versatility empowers designers to integrate the desired visual statement into the final product seamlessly.
Enhanced Durability with a Protective Barrier
Unlike uncontrolled rusting, which weakens the metal, the controlled oxidation process creates a stable oxide layer. This layer acts as a protective barrier, shielding the underlying steel from further corrosion and environmental degradation, ultimately extending its lifespan.
Low-Maintenance Appeal for Long-Term Applications
Oxidized steel offers a significant advantage over traditional painted finishes – minimized maintenance requirements. The naturally occurring oxide layer eliminates frequent touch-ups, making it a practical choice for long-term applications.
Evolving Character Through Natural Refinement
Oxidized steel exhibits a unique characteristic – it gracefully evolves over time. Natural exposure to the elements can further refine the patina, adding depth and character to the finish. This creates a living material that matures alongside its environment, fostering a sense of organic beauty.
Environmentally Sustainable Considerations
Several oxidizing techniques favor environmentally friendly methods, minimizing harsh chemicals. The finish’s inherent durability also translates to a longer product lifespan, reducing the need for replacements and associated environmental burdens.
Preparing Steel for Oxidation: Essential Steps
Effective and consistent application of controlled oxidation techniques necessitates meticulous pre-treatment of the steel substrate. This pre-treatment stage directly influences the final oxide layer’s uniformity, adherence, and aesthetic qualities.
The following procedures are crucial for optimal results:
Degreasing and Decontamination
Employ appropriate degreasing solvents to thoroughly remove surface contaminants such as oils, grease, and machining residues. For persistent contaminants, consider utilizing mechanical cleaning methods like abrasive blasting or controlled sanding with a specified grit size.
Inhibition Removal
Certain mill oils or protective coatings applied during steel production can impede oxidation. To eliminate these inhibitions and ensure optimal surface reactivity, utilize designated chemical solutions or controlled mechanical techniques.
Surface Homogeneity
Achieve a uniform surface texture across the entire steel substrate. Uneven surfaces can lead to inconsistencies in the oxidation process and the resulting oxide layer. Implement controlled sanding or grinding techniques with a specified grit size to achieve a consistent surface profile.
Abrasion Management
While a slightly roughened surface can promote even oxidation, excessive abrasion can negatively impact the final finish. Maintain a precisely controlled level of surface roughness through a meticulous selection of abrasive grits or cleaning methods.
Cleaning and Desiccation
After pre-treatment, thoroughly clean the steel with clean, demineralized water to remove any residual cleaning agents or debris. Ensure complete drying through a designated drying process to prevent the formation of unwanted flash rust before initiating the desired controlled oxidation process.
Adhering to these rigorously defined pre-treatment protocols lays the foundation for a successful and controlled oxidation process, ultimately yielding a visually captivating and functionally superior final product.
Common Chemicals Used to Oxidize Steel
Choosing chemical agents plays a significant role in the process when it comes to achieving unique and striking oxidized steel finishes. Different chemicals can produce varying colors, textures, and oxidation levels on steel surfaces. Here are some common chemicals used in the oxidation of steel:
Liver of Sulfur (Potassium Sulfide)
This water-based solution exhibits a high reactivity with iron in steel, readily forming a dark brown to black iron sulfide film. Metal artisans favored it for achieving a deep black patina with a smooth, almost lacquered appearance.
Salts (Ferric Chloride, Sodium Chloride)
Aqueous solutions containing specific salts, such as ferric chloride or sodium chloride, act as catalysts for forming a mottled, rusty texture. The precise salt used and its concentration directly influences the color and pattern of the resulting oxide layer, allowing for precise control over the final aesthetic.
Acids (Citric Acid, Phosphoric Acid)
Diluted solutions of mild acids, like citric or phosphoric acid, create a textured, aged appearance on the steel surface. The etching effect of these acids strategically exposes underlying iron layers to further oxidation, resulting in a variegated finish with a controlled level of detail.
Patination Accelerators
These commercially available solutions, often formulated by leading metal finishing suppliers, comprise a balanced blend of mild chemicals and catalysts. They expedite the controlled oxidation process, allowing for the faster achievement of desired patinas within a controlled environment.
Pre-mixed Metal Patinas
Pre-mixed metal patinas offer a curated range of color options for a more user-friendly approach to specific applications. These solutions typically contain a combination of carefully selected oxidizing agents and coloring pigments, simplifying the application process and ensuring consistent results for professional metalworking projects.
It’s crucial to note that the effectiveness of these chemicals can vary depending on the specific steel type and the desired final finish. Experimentation and testing on a small, inconspicuous area of the steel are highly recommended before full-scale application to ensure optimal results.
Techniques for Applying Oxidizing Agents to Steel
The careful application of oxidizing agents plays a pivotal role in achieving oxidized steel finishes’ desired aesthetic and functional properties. Here’s a breakdown of some commonly used techniques:
- Immersion:
- Submerge the cleaned and prepared steel substrate entirely in a tank containing the chosen oxidizing agent solution.
- This method ensures uniform coverage and consistent patinas across large, flat surfaces.
- Agitation of the solution or the steel piece can promote even distribution of the oxidizing agent.
- Wiping/Brushing:
- Apply the oxidizing agent solution directly onto the steel surface using a clean cloth, sponge, or brush.
- This technique allows for targeted application and the creation of textured or patterned effects through controlled brushstrokes.
- Multiple coats might be necessary to achieve the desired level of oxidation.
- Fumigation:
- Enclose the steel in a chamber containing a controlled atmosphere saturated with the vapors of the oxidizing agent.
- This method is particularly effective for achieving a light, even patina on three-dimensional objects.
- Careful temperature and exposure time monitoring are crucial to prevent excessive or uneven oxidation.
- Spraying:
- Utilize a dedicated spray applicator to apply the oxidizing agent solution onto the steel surface.
- This technique offers a degree of control over application thickness and faster coverage on large areas than manual wiping.
- Overspray and proper ventilation need careful consideration.
- Electrolytic Patination:
- This specialized technique involves submerging the steel anode in a solution containing the oxidizing agent and applying a controlled electrical current.
- The precise control over the electrical parameters allows for highly uniform and consistent patination, particularly beneficial for intricate details or mass production applications.
The choice of technique depends on several factors, including:
- Desired Finish: Different techniques create varying textures and uniformity levels.
- Project Complexity: Immersion is ideal for flat surfaces while spraying might be faster for large areas.
- Safety Considerations: Fumigation necessitates proper ventilation while spraying requires overspray control.
Always consult the recommended application procedures for the specific oxidizing agent being used. Experimentation and testing on a small, inconspicuous area of the steel are highly recommended before full-scale application.
Post-Oxidation Treatments for Longevity and Protection
After creating a stunning oxidized steel surface, implementing a series of post-oxidation treatments becomes paramount. These treatments solidify the finish’s aesthetic and functional integrity, ensuring its enduring beauty and performance.
Neutralization
Residual oxidizing agents remaining on the steel substrate after oxidation can induce further, uncontrolled reactions. A meticulously formulated neutralization solution, often a mild alkaline solution like sodium bicarbonate (baking soda), is employed to mitigate this. This solution effectively halts further oxidation, fostering a stable and predictable finish.
Following this application, thorough rinsing with demineralized water removes any residual neutralizing agent, ensuring optimal surface preparation for subsequent treatments.
Meticulous Drying
The presence of moisture can negatively impact the newly formed oxide layer. Therefore, it is critical to ensure complete drying of the steel surface following the neutralization step. This can be achieved by implementing controlled drying processes, such as forced air circulation or designated dehydrating chambers.
This meticulous approach safeguards against the formation of flash rust or any other moisture-related complications that could compromise the integrity of the finish.
Optional: Enhancing Durability with a Protective Wax Coating
While the established oxide layer provides some protection, a thin, microcrystalline wax coating can further bolster the finish’s longevity. This additional layer is a hydrophobic barrier, significantly minimizing moisture contact and slowing environmental degradation.
When opting for this approach, selecting a wax specifically formulated for metal applications is crucial to ensure optimal compatibility with the oxide layer’s unique characteristics.
Establishing Long-Term Maintenance Protocols
One significant advantage of oxidized steel finishes is their inherently low-maintenance requirements compared to traditional painted surfaces. Regular cleaning with a gentle soap solution and demineralized water removes dust and surface contaminants, preserving the finish’s aesthetic appeal. It’s crucial to avoid abrasive cleaning methods or harsh chemicals, as these can potentially damage the delicate oxide layer and compromise its protective qualities.
Final Thoughts
Creating oxidized steel finishes is a sophisticated process that blends art and science. It requires an in-depth understanding of chemical reactions and meticulous attention to detail.
With the ability to create a wide range of colors, textures, and patterns, oxidized steel finishes offer endless possibilities for customization and creativity in various industries. Whether for architectural elements, interior design, automotive parts, or industrial equipment, this unique finishing process adds a touch of uniqueness and sophistication to any project.
At Valence Surface Technologies, we understand the importance of quality surface finishing in the aerospace, defense, space, and satellite industries. As the world’s largest independent aerospace product finishing company, we are committed to providing top-of-the-line services that meet the highest standards of excellence and durability.
Contact us today. Our team of experts is here to discuss your specific requirements and explore customized surface finishing solutions that meet your needs.
Read also:
- Steel Forging For Enhanced Aerospace Performance Is It Worth It?
- Adhesive Primer: Essential For Aerospace Surface Finishing
- Best Practices For Electroplating In Aerospace Applications
Frequently Asked Questions
What’s the difference between natural and artificial oxidation?
Natural oxidation occurs over time as steel is exposed to atmospheric conditions, leading to a rust layer that provides a distinctive appearance but may compromise the metal’s integrity if not controlled. Artificial oxidation, on the other hand, is a controlled process where oxidizing agents are applied to the steel surface to accelerate the oxidation process, creating a desired finish more quickly and with potential additional protection against further corrosion.
What are some considerations for maintaining and protecting oxidized steel?
Maintaining oxidized steel involves regular inspections to check for any signs of excessive corrosion that might compromise the material’s integrity. It’s also crucial to apply protective coatings or sealants designed for oxidized surfaces to protect the finish from environmental elements and to preserve its aesthetic appeal. Avoiding harsh chemicals and abrasives that can damage the patina is also essential.
What safety measures should be taken when working with oxidizing agents?
Safety is paramount when handling oxidizing agents, as they can be hazardous. Always wear protective gear, including gloves, goggles, and appropriate clothing, to prevent skin and eye contact. Work in a well-ventilated area to avoid inhaling fumes, and ensure that you have suitable fire extinguishing measures in place since some agents are highly flammable or can intensify a fire.
What type of steel is best suited for oxidation?
Most types of steel can be oxidized, but the results can vary based on the alloy composition. Mild steel is commonly used for oxidized finishes due to its affordability and susceptibility to oxidation. However, alloys with a higher corrosion resistance may not oxidize as easily or predictably, affecting the final appearance.
How does the cost of oxidized steel finishes compare to other options?
Oxidized steel finishes can be cost-effective compared to high-end finishing options like electroplating or powder coating, mainly if artificial oxidation is used, as it speeds up the process. However, the cost can vary depending on the project’s complexity, the size of the surfaces being treated, and the required level of protection and maintenance.
How long does it take to oxidize steel?
The time to oxidize steel can range from a few hours to several days, depending on the desired finish and the method used. Natural oxidation is a slower process that can take months or even years, while artificial oxidation methods can achieve a similar appearance in a much shorter timeframe.
What industries use oxidized steel?
Oxidized steel is used across various industries, including aerospace, automotive, architecture, and interior design. Its unique aesthetic and protective qualities make it suitable for exterior façades, decorative elements, and components requiring a certain degree of corrosion resistance.
Can you reverse the oxidation process on steel?
While it’s difficult to reverse oxidation once it has occurred completely, certain methods can remove rust and restore the steel surface to some extent. Physical abrasion, chemical treatments, and electrochemical processes can remove the oxidized layer, but these methods may not always restore the steel’s original appearance or properties.
Can I paint over an oxidized steel finish?
Painting over an oxidized steel finish is possible, but proper surface preparation is crucial. The oxidized layer must be stable and adequately sealed with a primer designed for use on rusted or oxidized surfaces to ensure good paint adhesion and to prevent further oxidation under the paint layer.
How can I tell if rust on my steel is simply cosmetic or indicates a structural problem?
Determining whether rust is merely cosmetic or indicative of a structural issue involves assessing the extent and depth of the corrosion. Surface rust is generally cosmetic and can be treated, but if it has penetrated deeply into the steel, compromising its thickness and strength, it indicates a structural problem that requires professional evaluation.