Everything from building to transportation to kitchenware. Everywhere you look, stainless steel is present. The material’s apparent endurance, performance, and exceptional strength make it the ideal choice for a range of applications.
Yet, corrosion still happens very slowly on stainless steel. Stainless steel passivation is one procedure that all types of stainless steel parts must go through. The alloy has numerous intrinsic qualities that keep it safe from corrosion.
In this article, we will discuss the basics of stainless steel passivation, including what it is, how it works, and the benefits it provides. We will also discuss the passivation process, standards, and specifications, as well as answer some frequently asked questions. By the end of this article, you should have a better understanding of stainless steel passivation and how it can help protect your products.
What Is Stainless Steel Passivation?
Stainless steel passivation is a process that improves the corrosion resistance of stainless steel by removing free iron particles from its surface.This is accomplished by coating the steel with a mild acid solution, which aids in the formation of a protective oxide layer on its surface. This layer functions as a barrier to stop additional rusting. Passivation is a crucial component of stainless steel maintenance that contributes to the material’s performance and life extension.
The process of passivation involves cleaning the surface of the stainless steel to remove any dirt, oils, and other contaminants that can interfere with the passivation process. Often, a mild detergent and water solution is used for this.
The steel is then treated with a moderate acid solution to eliminate any leftover free iron particles after the surface has been thoroughly cleaned. Further corrosion is aided by the protective oxide layer that the acid solution forms on the stainless steel’s surface.
How Does Passivation Work?
The process works by forming a protective oxide layer on the surface of the steel. This layer aids in preventing corrosive substances like oxygen and water from coming into touch with the steel.
Steel is submerged in an acidic solution during the passivation process, often nitric acid. This mixture aids in cleaning the steel’s surface of any impurities and oxides. Moreover, the acid aids in the formation of a thin oxide layer on the steel’s surface. This layer serves as a shield between the steel and the outside world, preventing corrosion.
Once the passivation process is complete, water removes any remaining acid. This makes it easier to make sure that no acid residue, which might lead to corrosion, is left on the steel’s surface.
Passivation Types
Chemical Passivation
Chemical passivation is one of the most common types of passivation. This procedure removes the free iron atoms from the steel’s surface using a chemical solution. Usually, nitric acid, hydrofluoric acid, or citric acid make up this solution.
The steel is coated with the chemical solution, which is then allowed to soak there for anything from 15 minutes to 2 hours. The solution is removed following the soak, and the steel is then washed with water.
Electrochemical Passivation
Electrochemical passivation is a different kind of passivation. In this procedure, the surface of the steel is cleaned of loose iron particles using an electrical current. Components made of stainless steel that are too large to be immersed in a chemical solution are frequently passivated using this method.
The steel is exposed to the electrical current for a length of time, typically between 30 minutes and 4 hours, before being allowed to passivate. The steel is washed with water when the passivation is finished.
Mechanical Passivation
Mechanical passivation is the third kind of passivation we’re going to discuss. In this procedure, the surface of the steel is cleaned of loose iron particles using mechanical techniques.
Components of stainless steel that are too small to be immersed in a chemical solution are frequently passivated using this method. The steel is subjected to the mechanical process, which is then allowed to passivate for a period of time, often lasting between 15 minutes and 4 hours. The steel is washed with water when the passivation is finished.
The removal of free iron particles from the surface of the steel, which increases corrosion resistance and enhances overall performance, is the same regardless of the passivation process type employed.
Why Passivate Stainless Steel?
Stainless steel is a popular material for a wide range of applications due to its durability and strength. However, it is vulnerable to corrosion and rusting if not properly maintained. Passivation is a process that helps to protect stainless steel from corrosion and rusting.
Iron’s Vulnerability To Corrosion
Iron corrodes when it is exposed to atmospheric oxygen and moisture. The iron oxidizes and forms rust as a result of the electrochemical reaction this combination produces. A reddish-brown material called rust can corrode and weaken iron, causing structural harm and even failure.
To protect iron from corrosion, it is important to use a protective coating or treatment. One of the most popular techniques for preventing corrosion in iron is passivation. The passivation process involves applying a chemical or acid solution to the iron’s surface to generate a layer of protection. This layer keeps moisture and oxygen from getting to the iron, stopping corrosion.
Chromium Prevents Corrosion
Chromium is a key element in stainless steel that helps to prevent corrosion. A thin, undetectable layer of chromium is present on the surface of stainless steel. This layer, often referred to as the passive layer, serves as a barrier to keep oxygen and water away from the steel. The steel is thereby shielded from rusting and corrosion.
Chromium generates a chromium oxide coating on the surface of the steel when it interacts with oxygen and water. This film offers an additional layer of protection and is very corrosion resistant. The steel’s appearance or functionality is unaffected by the very thin chromium oxide coating.
As long as the stainless steel is exposed to oxygen and water, the passive layer will continue to regenerate. This makes sure that the steel is always shielded from rust and corrosion. Also, since the steel does not need any extra cleaning or care to stay in good condition, the chromium in the steel contributes to a reduction in overall maintenance costs.
Contaminants In The Manufacturing Process
Corrosion in stainless steel components can be primarily caused by contaminants in the production process. The raw materials used to create the components, the production environment, and even the tools and equipment utilized throughout the manufacturing process are some of the possible sources of these contaminants.
In order to prevent corrosion from these contaminants, it is important to ensure that all components are thoroughly cleaned prior to passivation. This entails washing the parts with water after cleaning them using a solvent, such as isopropyl alcohol.
Components should also be checked for any lingering pollutants and cleaned once more if necessary. Repeat this procedure until all pollutants have been eliminated. The components can then be passivated to prevent corrosion after being cleaned.
Improved Machinability
One of the main advantages of passivating stainless steel is improved machinability. Passivation clears impurities from stainless steel’s surface, which can result in better machinability. A protective oxide layer is created on the surface of the steel during the passivation process, acting as a shield to stop corrosion.
This oxide layer also improves cutting and lessens tool wear by reducing friction between the steel and the cutting tool. Moreover, passivation can lessen the chance of tool breakage, improving machinability and lengthening tool life.
After passivation, stainless steel has better machinability, increasing manufacturing efficiency and reducing costs.
Benefits Of Steel Passivation
Steel passivation is a process used to enhance the corrosion resistance of stainless steel products. This process involves the removal of free iron particles from the surface of the stainless steel, which can lead to corrosion if not removed.
The passivation process also helps to restore the chromium oxide layer, which is the main component that gives stainless steel its corrosion resistance. Several benefits are associated with steel passivation, including improved machinability, increased corrosion resistance, and improved appearance.
When To Passivate Steel
Knowing when to passivate stainless steel is essential in order to get the best results and ensure that the product will last for years.
Passivating stainless steel as soon as possible after it has been manufactured or machined is important. This is because when steel is freshly machined, the surface is exposed to the elements, making it more susceptible to corrosion. The surface is sealed and shielded from oxidation with the use of passivation.
Passivation should also be performed when the stainless steel has been exposed to any kind of pollutant, such as oil, grease, or dirt. If these pollutants are not eliminated, corrosion and rust may result. Passivation aids in clearing any pollutants from the steel’s surface and guards against future deterioration.
Finally, passivation should be done when the stainless steel has been exposed to a high temperature. Steel may become brittle and more corrosive at high temperatures. Passivation aids in regaining the steel’s original characteristics and guards against further deterioration.
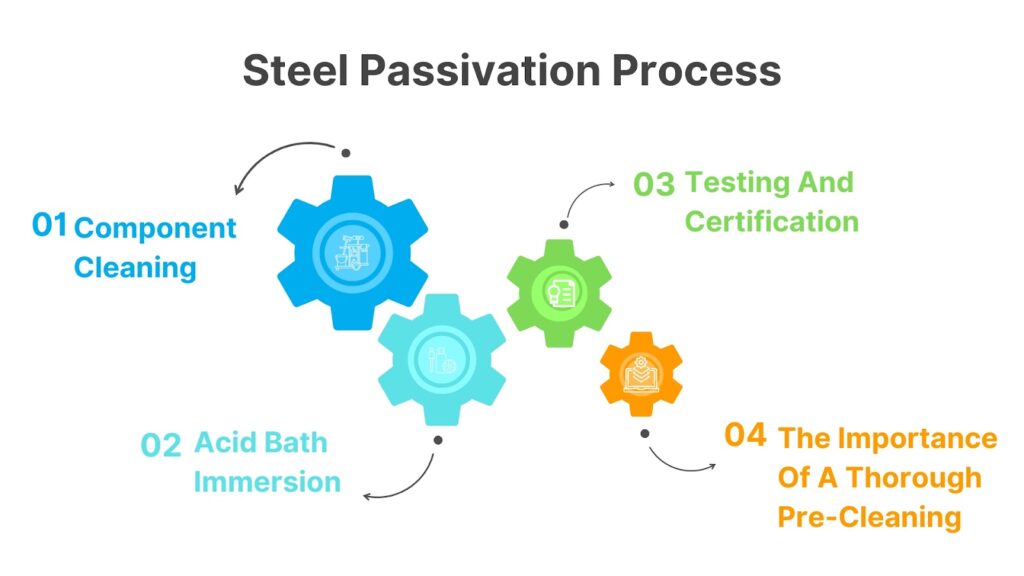
Steel Passivation Process
Steel passivation is the process of treating the surface of stainless steel to remove contaminants and improve its corrosion resistance. The process involves immersing the stainless steel parts in an acid solution and then rinsing them with water. This process can be done by hand or through an automated system.
Component Cleaning
Component Cleaning is an important step in the stainless steel passivation process. It entails cleaning the stainless steel’s surface of any debris, oils, and other impurities before the passivation procedure starts. This step is essential for the passivation process to be successful and for the stainless steel to be adequately shielded from rust and corrosion.
Chemical cleaning, which involves using a specialized cleaning solution to remove any dirt, oils, or other impurities from the surface of the stainless steel, is the most popular technique for component cleaning. This cleaning solution, which is typically acidic or alkaline, is made to disintegrate and remove any impurities from the surface of stainless steel.
Once the stainless steel has been cleaned, it is then rinsed with water to remove any remaining cleaning solution. After this step, the stainless steel is ready for the passivation process.
It is important to note that component cleaning is not the same as passivation. Component cleaning is only the first step in the passivation process, and it is necessary to ensure that the passivation process is effective. Without proper component cleaning, the passivation process may not be effective, and the stainless steel may not be properly protected from rust and corrosion.
Acid Bath Immersion
Acid bath immersion is a key part of the stainless steel passivation process. It entails placing the stainless steel parts in an acid bath for a predetermined amount of time. This procedure aids in the removal of any impurities from the metal’s surface and aids in the formation of a passive layer. Nitric acid solution is typically the acid used in the procedure, but other acids may be used as well, depending on the type of metal being treated and the desired outcomes.
After the components have been removed from the acid bath, they are then inspected for any signs of corrosion or other damage. The components are prepared for the following stage of the passivation process if they are discovered to be in good condition. Before the passivation procedure may continue, the components must be fixed if any damage is discovered.
Testing And Certification
Testing and certification are essential to ensuring that stainless steel passivation is carried out correctly and to the highest standards. Testing and certifying the process is important to ensure that the passivation is effective and that the stainless steel components have been adequately protected against corrosion.
Once the components have been tested and certified, the process is complete and the components are ready for use. It is important to remember that the passivation process can only be used on components made of stainless steel that have been correctly cleaned and prepared. The components may still be susceptible to corrosion if any impurities or pollutants are present after the passivation procedure.
It is also important to note that the passivation process should be carried out by a qualified technician who is familiar with the process and the applicable standards and regulations. This guarantees the components are properly protected during the process and that it is carried out correctly.
Additionally, the technician must be able to certify that the procedure was carried out accurately and in accordance with all relevant standards and laws.
The Importance Of A Thorough Pre-Cleaning
Pre-cleaning is an important step in the passivation of stainless steel. Prior to passivation, it is important to thoroughly clean the stainless steel to ensure the best possible results. Contaminants, including grease, oil, and dirt, are eliminated during the cleaning process so that the passivation process won’t be hampered. As a result, stainless steel is better protected against corrosion and the passivation process is more effective.
Depending on the impurities present, various techniques may be used during the pre-cleaning process. For instance, if the stainless steel is covered in dirt and grime, it might need to be pressure washed or scrubbed manually. If there is oil or grease, it might need to be cleaned using chemicals like an alkaline cleaner or degreaser. Sandblasting, ultrasonic cleaning, and electrocleaning are some additional techniques.
Passivation Standards And Specifications
Passivation standards and specifications are used to ensure that stainless steel products are properly passivated and meet the highest quality standards. The American Society for Testing and Materials (ASTM) has established several standards for stainless steel passivation. These include ASTM A380, ASTM A967, ASTM A967-05, ASTM A967-09, ASTM A967-11, and ASTM A967-14.
ASTM A380 is the standard for cleaning, descaling, and passivating of stainless steel products. It covers the requirements for the cleaning and passivating of stainless steel in a solution of nitric acid and water. This standard also covers the requirements for the passivation of stainless steel in a solution of nitric acid and chromic acid.
ASTM A967 is the standard for chemical passivation treatments for stainless steel parts. It covers the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and water. This standard also covers the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and chromic acid.
The ASTM A967-05, ASTM A967-09, ASTM A967-11, and ASTM A967-14 standards cover the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and citric acid. The standards cover the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and sodium dichromate.
The ASTM A967-09 and ASTM A967-14 standards also cover the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and sodium nitrate.
The ASTM A967-09, ASTM A967-11, and ASTM A967-14 standards also cover the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and sodium nitrite.
The ASTM A967-09, ASTM A967-11, and ASTM A967-14 standards also cover the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and hydrofluoric acid.
The ASTM A967-09 and ASTM A967-14 standards also cover the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and sodium hydroxide.
The ASTM A967-09, ASTM A967-11, and ASTM A967-14 standards also cover the requirements for the chemical passivation of stainless steel parts in a solution of nitric acid and sodium hypochlorite.
These standards provide detailed information about the requirements for the cleaning and passivation of stainless steel products. They provide guidance on the types of materials that can be used for passivation, the types of solutions that can be used, and the conditions that must be met in order to achieve the desired results. They also provide guidance on the testing and certification of passivated stainless steel products.
Final Thoughts
Passivation is a crucial procedure that keeps stainless steel from corroding and keeps it looking brand new. Therefore, it is unquestionably an effective way to increase productivity and service quality.
Even though the procedure is straightforward, common passivation can be completed at home with a basic kit. You must work with a reputable business if you want production-grade stainless steel passivated parts.
Valence Surface Technologies is a full-service surface finishing company specializing in the commercial aerospace, defense, space and satellite industries. With ten strategically located sites across the United States, Valence provides a start to finish solution from NDT and chemical processing (including steel passivation), to paint and sub-assembly.
Do you have a metal finishing project? We are happy to give you a quote that’ll put you on the track to the success of that project. Request a quote here
FAQs
What’s involved in the passivation validation process?
The stainless steel surface is tested as part of the passivation validation process to make sure it has been sufficiently passivated. This is accomplished by measuring the quantity of chromium on the steel’s surface and looking for any free iron. The passivation process has been successful if the chromium levels are within the desired range and there is no free iron present.
Why are automated systems easier to process validate than manual equipment?
Automated systems are easier to process and validate because they are more consistent and can be programmed to adhere to specific passivation parameters. As a result, it is simpler to guarantee that the stainless steel has been passivated sufficiently because the automated system may be set to adhere to the necessary criteria.
Is passivation the same as pickling?
No, passivation and pickling are two different processes. Passivation is a chemical process that is used to increase the corrosion resistance of stainless steel, while pickling is a mechanical process that is used to remove surface contaminants.
Does passivation make stainless steel corrosion proof?
No, passivation does not make stainless steel corrosion proof. It does, however, increase the corrosion resistance of the steel and can help to protect it from rust and corrosion.
Is the passivation of stainless steel optional?
No, the passivation of stainless steel is not optional. It is an essential part of the manufacturing process and is necessary to ensure that the steel is adequately protected from rust and corrosion.
What is the best way to passivate stainless steel?
The best way to passivate stainless steel is to use a chemical process that involves an acid bath immersion. This process helps to remove contaminants from the surface of the steel, as well as increasing the chromium content on the surface of the steel.
What are the disadvantages of passivation?
The main disadvantage of passivation is that it can be expensive and time consuming. Additionally, it is important to ensure that the passivation process is done correctly, as improper passivation can result in reduced corrosion resistance.
How can you tell if stainless steel is passivated?
You can tell if stainless steel is passivated by testing the surface of the steel. This is accomplished by measuring the quantity of chromium on the steel’s surface and looking for any free iron. The passivation procedure has been successful if the chromium levels are within the desired range and there is no free iron present.
What acid is used for passivation?
The acid used for passivation depends on the type of stainless steel being passivated. Common acids used for passivation include nitric acid, citric acid, and hydrofluoric acid.
What is the pH for passivation?
The pH of passivation depends on the type of acid used for the process. Generally, the pH should be between 1.0 and 2.5 for nitric acid, 2.0 and 3.0 for citric acid, and 1.0 and 2.0 for hydrofluoric acid.