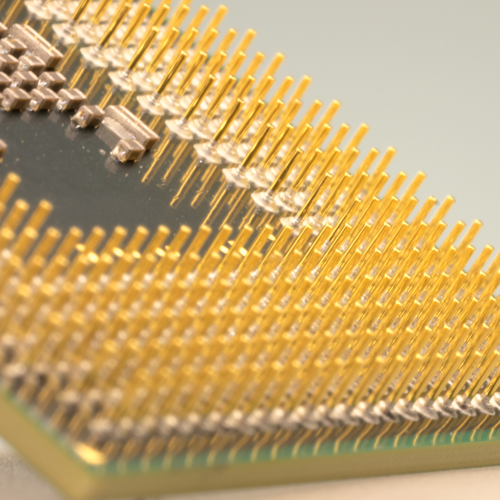
Gold Plating
Gold plating advantages include good corrosion resistance, good solderability, and, when alloyed with cobalt, it has very good wear resistance. Gold is a precious metal, which means that it will not oxidize in air, so its electrical conductivity stays uniform over long periods.
Gold is commonly used in electrical switch contacts, connector pins and barrels, and other applications where intermittent electrical contact occurs. It is also used as a radiation shield, in infrared reflectors and electronic satellite housings. These and other advantageous properties are among the reasons why gold plating volume continues to grow, even though the material’s price has risen dramatically over the years.
Gold’s hardness varies with the purity of the deposit, with the purest gold plating providing the softest deposit, and the lowest purity deposit being the hardest. Even though hard gold deposits are lower in purity than the soft gold, all gold plating at Valence Surface Technologies is 24 karat gold.
Valence Surface Technologies has three types of gold plating baths to serve our customer’s needs:
99.9% gold deposits with a knoop hardness of 90 Knoop Hardness maximum, used for specific types of wire bondability and solderability where high purity is required to achieve proper adhesion strength of the bond and where the coating will be exposed to very high temperatures. Commonly called a Type 3 gold.
99.7% gold deposits with a Knoop hardness range of 130–200 Knoop Hardness. This is a good overall deposit used for wear resistance, solderability, wire wrap, and many others. The gold
99.0% gold deposits are primarily used for wear resistance. The hardness of this deposit is 201 Knoop Hardness minimum. Due to stresses developed when the coating is hardened, this deposit can be plated only in limited thicknesses. Please contact us with any questions regarding the maximum thickness of this deposit before issuing a purchase order. This deposit is commonly called Type 2 Gold.
Valence Surface Technologies gold plates many different types of parts made of many different materials; In some cases, the parts have several different finishes on them requiring close tolerance masking.
Request a quote for the gold plating you need, or contact us to find out why gold plating is a good fit for your application.

Gold Plate Process

Gold Plate Black Anodize Process
Gold is considered malleable, practically indestructible, and completely recyclable while also being virtually immune to the effects of water, air, and oxygen. It is a crucial component in many industrial, aerospace, medical, and electrical applications due to its distinctive combination of properties. Gold is one of the most electrically conductive metals and is also an excellent conductor of heat or thermal energy.
Gold plating is helpful for parts that need to remain flexible while also having wear or corrosion resistance. The element is non-reactive and has a high electrical conductivity. Even though it is the priciest electroplating option, it is by far the best way to ensure the worth and quality of your product.
In this article, we will discuss gold plating, why it is a good material for aerospace plating applications, gold plating specifications, and a breakdown of the gold plating process.
What Is Electroplating?
Before we talk about gold plating, it is important that we define what electroplating is. Electroplating is the process of coating one metal or metal object with a very thin layer of another metal, typically by applying a direct electric current. This partially dissolves the metals and creates a chemical bond between them. The coating applied by electroplating is usually around 0.0002 inches thick.
The plating alters the surface of the base metal permanently by forging a chemical bond. This implies that it won’t inevitably fall off or split apart. But over weeks, months, or even years of use and wear, the plating can come off.
This top layer has very particular uses because it is so thin. Because they provide additional protection against rust, damage, and corrosion, some metals are used as coatings.
What Is Gold Plating?
Gold plating is a process where a thin coat of gold is layered onto the surface of another base metal. This process was invented in 1805 by Luigi Valentino Brugnatelli, a famous Italian chemist who electroplated a thin layer of gold onto silver.
Until recently, gold plating was mostly used for decorative purposes – to add a luxurious touch to jewelry, watches, or other objects. However, gold plating is now used in various industries, including electronics and aerospace.
Importance Of Aerospace Plating
The majority of modern aircraft are made of titanium and titanium alloys. For aerospace crafts, titanium is highly valued because of its low density, high strength, and lightweight. Steel, aluminum, and magnesium are other regularly utilized metals.
Even though these metals are fundamental to the manufacturing of aircraft, they are not without flaws. These metals are nonetheless prone to oxidation, corrosion, and decomposition at high temperatures. Aircraft plating is utilized as a metal finishing procedure to shield aerospace metals from various risks to their integrity.
Electroplating and electroless plating are the two main plating techniques. By adopting a method called electrodeposition, electroplating entails covering a metal surface with ions of another metal.
Benefits of either type of aerospace plating include:
- Improved corrosion protection
- Enhanced strength of the metal substrate
- Increased resistance to high temperatures
- Component longevity
- Enhanced electrical conductivity
- Increased resistance to oxidation
- Aesthetic appeal
Why Gold Plating For Aerospace?
Gold plating is frequently used in the aerospace industry to enhance the performance of various parts and components. Gold plating is used in this industry primarily for its mechanical and electrical qualities.
It’s fascinating how gold is used in the aerospace sector. Even though we don’t often see walk-ins carrying shuttle parts while dressed in space suits, we thought you might be interested in learning more about it.
You wouldn’t think that since gold is a precious metal, humans would send it into space. But gold also has some special qualities that make it extremely valuable in the space program.
For instance, space suits make use of it. The suits astronauts wear in space are coated in a thin layer of gold to shield them from radiation and reflect and deflect the sun’s scorching heat. That is the glamorous function that gold plays in the aerospace sector.
Gold is also used in the circuitry. Gold will last a long time if you need parts that can function well without lubrication, upkeep, or repair. Because it is a dependable conductor and connector, gold is used in aeronautical circuitry. Additionally, many components are fitted with the gold-coated polyester film due to its reflective properties. This aids in stabilizing the temperature inside the aircraft by reflecting infrared radiation. Without this coating, the aircraft’s dark-colored components would absorb more heat, which would increase the rate of breakdown.
As a lubricant between mechanical components, gold is also employed. Organic lubricants break down in space due to their instability. Once more, this is brought on by the extreme radiation received outside of the Earth’s insulating atmosphere. Because gold is malleable, it can be processed into a very thin film that lubricates and coats important moving parts. This is due to the lubricant action of the gold molecules, which under frictional forces, slide past one another. It’s an intriguing use of gold that is uncommon in other contexts.
Gold plating is also used in aerospace applications for the following technical reasons:
Mechanical Properties
This includes characteristics like corrosion resistance, wear resistance, and reduced friction. The aerospace industry values all of these characteristics because they can help parts and components last longer and perform better overall.
Electrical Properties
This includes characteristics like resistance and conductivity. Gold has a very low resistance and is a superb electrical conductor. This makes it perfect for use in electronic components and other settings where resistance and conductivity are crucial.
Gold Plating Process
Step 1: Surface Preparation
Oils and other impurities must be removed from the metal’s surface before plating it, and the object must be polished. Surface preparation techniques can include sandblasting, tumbling, polishing, and more. Water, acid etch, alkaline cleaners, solvents, abrasives, or a combination of these can be used. Acid or non-acid ultrasonic baths and high-rpm rouge wheel polishing are two common cleaning techniques. Two factors make it necessary to do this:
- To increase compliance. (Dust and dirt prevent the plated metals from securely adhering to the jewelry item.)
- To keep impurities out of the plating tanks.
Step 2: Cleaning
Electrocleaning, ultrasonic cleaning, or steaming are typically used after the surface has been prepared and inspected visually. To ensure that the metal is free of oils and dirt, which helps to produce superior plating results, a second, more thorough cleaning step is required. Any remaining oils that managed to stick around during the polishing process are blasted away by steam cleaning. Pay close attention to jewelry that is highly detailed and has numerous crevices.
Step 3: Rinse
The piece is rinsed thoroughly with water to remove any cleaning agents.
Step 4: Strike
A thin layer of premium nickel plating is adhered to the base metal by a strike layer, also known as a flash layer.
Occasionally, a buffer layer needs to be applied between the plating and the underlying surface to improve their bonding. When making costume jewelry, the base metal would contaminate the gold plating tanks, so a different metal is plated first.
Additionally, when the base metal, such as copper, is known to atomically migrate outside of the gold layer to produce tarnish spots after plating, this step is used. The reactive base metal and the plated metal are separated by this strike step. This increases the bright gold plating’s lifespan.
Step 5: Rinse Again
After the strike layer has been applied, rinse the surface again with distilled water to help remove any impurities that may be present.
Step 6: Base Coat
If a base coat is applied before gold, it is typically nickel. On a single piece, the plating may be applied in multiple layers. For instance, a silver substrate usually has layers of copper, nickel, and gold deposited on top of it in a gold-plated silver article.
Step 7: Final Coating
With time, temperature and voltage carefully controlled, the piece is submerged into the plating solution to attract ions of gold or the final metal that will show on the surface. Voltage and temperature requirements vary depending on the metal.
The cathode bar, which is a pole with a negative electrical charge running through it, is used to hang the items to be plated. Negatively charged items include the jewelry fastened to the cathode bar. The electrical charge is applied to the jewelry when it is submerged in the tank, and the negatively charged jewelry draws in the positively charged ions that are already present. The solution bath contains positively charged metal ions. Metal jewelry is plated when the cathode bar is lowered into the bath.
Step 8: Final Rinse
Finally, rinse the gold-plated surface with distilled water for a final time to help remove any impurities that may be present, and hang the piece up to dry completely.
Gold Plating Specifications
In some instances, it may be necessary to perform gold plating to meet military or engineering specifications.
MIL-DTL-45204D
The MIL-DTL-45204D gold plating specification replaces the previous MIL-G-45204C standard and satisfies the needs of the U.S. Department of Defense and its various support agencies.
Based on the material’s purity, this specification categorizes different types of gold: A minimum of 99.7 percent gold is present in Type I, 99.0 percent in Type II, and 99.9 percent in Type III. The Knoop scale also has four hardness grades: Grade A (90 maximum), Grade B (91–129), Grade C (139–200), and Grade D. (201 and over).
Possible combinations for purity vs. hardness are the following:
- Type I: A, B, or C
- Type II: B, C, or D
- Type III: A only
There are also gold plating thickness standards under this military spec. There are eight classes corresponding to the acceptable minimum thickness:
- Class 00-0.00002
- Class 0-0.00003
- Class 1-0.00005
- Class 2-0.00010
- Class 3-0.00020
- Class 4-0.00030
- Class 5-0.00050
- Class 6-0.00150
Additionally, there are particular specifications for the underplating of the gold coating. An undercoating of nickel, copper or a Cu/Ni may be used to satisfy the contract requirements, depending on the application and environment. If not absolutely necessary, avoid using a silver or silver/copper underplate. No matter the underplating’s makeup, a soft gold strike must be applied on top of it before the final layer of gold is added to help with adhesion and lower the chance of contamination.
ASTM B488 Gold Plating Specification
Engineering applications use gold plating that complies with the ASTM B488 standard. Gold coatings that meet this specification are typically used to increase the substrate’s corrosion resistance and to stop tarnishing. This specification’s classes and types are identical to those in the MIL-DTL-45204D gold plating standard. There are some variations in the thickness requirements, though. The following seven thickness classes for gold plating are defined by ASTM B488:
- 0.25-0.25 um
- 0.50-0.50 um
- 0.75-0.75 um
- 1.0-1.0 um
- 1.25-1.25 un
- 2.5-2.5 um
- 5-5.0 um
With the exception of applications requiring a coating thickness of 5.0 um or more when working with substrates made of copper or a copper alloy, nickel is the material of preference for underplating. Nickel also acts as a brightener for the leveling layer, as a corrosion inhibitor in surface pores, as a barrier against tarnish creeps on the gold topcoat, and as a load-bearing underlayer for the contacting surfaces, among other functions that make it the best undercoating material.
The issue of this standard establishes an ASTM Type designation that is new involving purity, which is established for electrodeposited gold:
- Mass percent gold, minimum, excluding potassium, carbon, and nitrogen 99.70: New ASTM Type I, MIL-DTL-45204 Type I, Old ASTM Type 2
- Mass percent gold, minimum, excluding potassium, carbon, and nitrogen 99.00: New ASTM Type II, MIL-DTL-45204 Type II, Old ASTM Type 3
- Mass percent gold, minimum, excluding potassium, carbon, and nitrogen 99.90: New ASTM Type III, MIL-DTL-45204 Type III, Old ASTM Type 1
The hardness values are specified by ASTM Code:
- Knoop Hardness Range 90 HK25 maximum: ASTM Code A
- Knoop Hardness Range 91–129 HK25: ASTM Code B
- Knoop Hardness Range 130–200 HK25: ASTM Code C
- Knoop Hardness Range >200 HK25: ASTM Code D
When it comes to the relationship between purity and hardness for good commercial practice, the following combinations are fully representative:
- New ASTM I – Old ASTM Type 2 – Code A, B, and C
- New ASTM II – Old ASTM Type 3 – Code B, C, and D
- New ASTM III – Old ASTM 1 – Code A only
Additional Requirements
Other requirements for adhering to the MIL-DTL-45204D and the MIL-DTL-45204D gold plating specification include making sure the base metal is free of surface flaws and carrying out the proper pretreatment procedures before plating, as examples.
Unless specifically stated in the contract, avoid electroplating steel parts and components with a tensile strength of 220,000 psi. Removal of all plating salts from the substrate and checking the component for blisters that will cause the piece to fail are acceptable post-plating procedures.
Types Of Gold Plating
Hard gold plating and soft gold plating are the two main types of gold plating, with duplex gold being a combination of the two. The three main categories of gold plating services are briefly described below:
Hard Gold Plating
Hard plating is a process that increases the hardness and wears properties of the gold deposit by adding a small amount of an alloying element or compound. Iron and even arsenic have been used in addition to the frequently used cobalt or nickel.
The grain structure of hard gold deposits is more refined, giving the metal a brighter overall appearance. Applications where there is repeated engagement or sliding contact benefit from the increased hardness of hard gold plating.
Soft Gold Plating
Contrary to hard gold plating, soft gold is almost completely pure, with purities of 99.9% or higher. Soft gold has a less refined grain structure than hard gold, giving the deposit a maximum hardness of 90 Knoop. Since there are no alloying components to oxidize due to the high purity, soft gold is ideal for wire bonding or high-temperature applications.
For low-load, static contact designs like lapping connections, soft gold is also ideal. Due to its high purity and biocompatibility, soft gold is frequently chosen for medical applications.
Duplex Gold Plating
Duplex gold plating combines both hard and soft gold deposits to create a finish with the characteristics of both types of gold plating. For instance, to increase the wear properties of the overall gold plating, a layer of soft gold that is highly corrosion-resistant can be top-coated with a deposit of hard gold.
Due to the different grain structures between the two gold layers, duplex gold deposits also have less overall deposit porosity. When compared to single-layer gold deposits, this can offer improved corrosion resistance at a lower overall gold consumption.
Benefits Of Gold Plating
Some of the benefits of gold plating include:
Corrosion Resistance
Many THAAD and PAC-3 systems are now deployed by the U.S. Army in hot, humid environments, like those in Afghanistan right now and in Iraq in the past during the two Persian Gulf Wars. These humid environments can hasten corrosion and rust, which can damage or incapacitate systems.
IIn addition to creating a barrier between the missile parts and the substrate, gold plating will help ensure more reliable connections, extending the life of the parts.
Heat Resistance
Gold is much better at dissipating heat than other metals, so contacts with gold plating can withstand much higher temperatures than contacts with another metal plating. The truth is that gold-plated defense parts can withstand exposure to temperatures of up to 125 degrees Celsius (257 degrees Fahrenheit).
Friction And Wear Resistance
Most ground and air defense systems have a large number of moving parts, all of which come into contact with one another at some point during their operational lifetime. This causes friction and wear. Equipment may need to be retired early due to severe wear from this type of contact because it is no longer able to function.
Any two pieces will experience less friction when they come into contact with one another thanks to the use of a gold coating, which will significantly increase the system’s overall working lifespan.
Malleability
Gold is one of the most malleable and pliable metals currently used in manufacturing. Due to its softness, it deforms when under pressure. While at first glance, this may not seem like a benefit, it is very useful in a field where you frequently deal with the coexistence of two or more metal types in a conductor, socket, or pin.
Gold can increase the area of contact between itself and another metal by deforming under pressure, which lowers electrical resistance.
Appearance
The product’s appearance may be crucial, depending on the item and how it will be used. The benefits mentioned above can be provided to the product while also improving its appearance by plating it with gold.
FAQs
How Thick Should Gold Plating Be?
The intended use of the component to be plated will determine the appropriate gold plating thickness. Keeping the plating thickness as low as practical is a good place to start when figuring out the overall thickness of the plating, despite the recent drop in the price of gold. The AMP report states that “in general, a hard gold coating of 0.8 microns (30 micro inches) over a minimum of 1.3 microns (50 micro inches) of nickel gives a degree of durability considered adequate for most connector applications.
Only applications where there is a low risk of fretting should use thin coatings of 0.03 to 0.1 microns (1 to 4 micro inches) of hard or soft gold over nickel underplate. Increasing the thickness of a gold coating tends to decrease the porosity, which reduces the contacts’ vulnerability to pore corrosion.
Does Gold Plating Tarnish?
The answer to this question is a bit complicated. Gold is a noble metal that doesn’t tarnish, as was already mentioned. The other metals (such as copper, silver, nickel, etc.) that are frequently used in gold plating are not as resistant to tarnishing.
These metal molecules slowly seep into the gold’s “thin” layer, breaking down the gold over time. When the base metal eventually touches the gold coating and comes into contact with oxygen and air, which cause tarnishing, the entire gold-plated object tarnishes.
How Long Does Gold Plating Last?
The thickness of the gold coating and the environment that the gold-plated item is exposed to will both affect the answer to this question. A thicker gold coating typically has a longer lifespan than a thinner one. This is due to the thicker coating’s increased resistance to abrasion, corrosion, and chemical attacks.
However, the environment that the item is exposed to will also have an impact on how long the gold coating lasts. For instance, a gold-plated object exposed to salt water or other corrosive substances won’t last as long as one used in a controlled environment. It’s crucial to remember that gold plating is not a long-term fix. Over time, the gold coating will eventually wear away, exposing the underlying metal. Also, it fades and loses its luster with time.
But generally, with proper care and maintenance, gold plating can last up to 2 years.
What Is the Grade Of Gold Used?
There is no one-size-fits-all answer to this question, as the grade of gold used for plating depends on the application.
For example, while pure gold (24 karats) is often used in the semiconductor industry, where a high level of conductivity is required, lower grades of gold – like 18 karats – are often used for plating jewelry because they are more durable.
Valence Has 3 types Of Gold Plating Baths To Serve Our Customer’s Needs
99.9% Gold Deposits with a maximum Knoop hardness of 90, are used for particular types of wire bondability and solderability, where high purity is necessary to achieve the proper adhesion strength of the bond and where the coating will be exposed to extremely high temperatures. Usually referred to as a Type 3 gold.
99.7% Gold Deposits with a Knoop hardness range of 130–200. This is a good all-around deposit used for wire wrapping, solderability, wear resistance, and many other purposes. The gold finish that is most frequently plated at Valence shares many characteristics with the harder finish, and its solderability is comparable to that of pure gold. Usually referred to as a Type 1 gold.
99.0% Gold Deposits are primarily used for wear resistance. The hardness of this deposit is 201 Knoop Hardness minimum. Due to stresses developed when the coating is hardened, this deposit can be plated only in limited thicknesses. Please contact us with any questions regarding the maximum thickness of this deposit before issuing a purchase order. This deposit is commonly called Type 2 Gold.
Industry Leaders In Aerospace Metal Finishing
There you have it – all the information you need to know about gold plating! But that’s not all from Valence. We also offer a range of other plating services, including cadmium plating, nickel plating, copper plating, and more. Check out our plating services to learn more.
Also, if you have any questions or need help with nickel plating services, our experienced team is here to help. Give us a call and we’ll get back to you as soon as possible. With Valence Surface Technologies, you can rest easy knowing your parts are in the best hands.